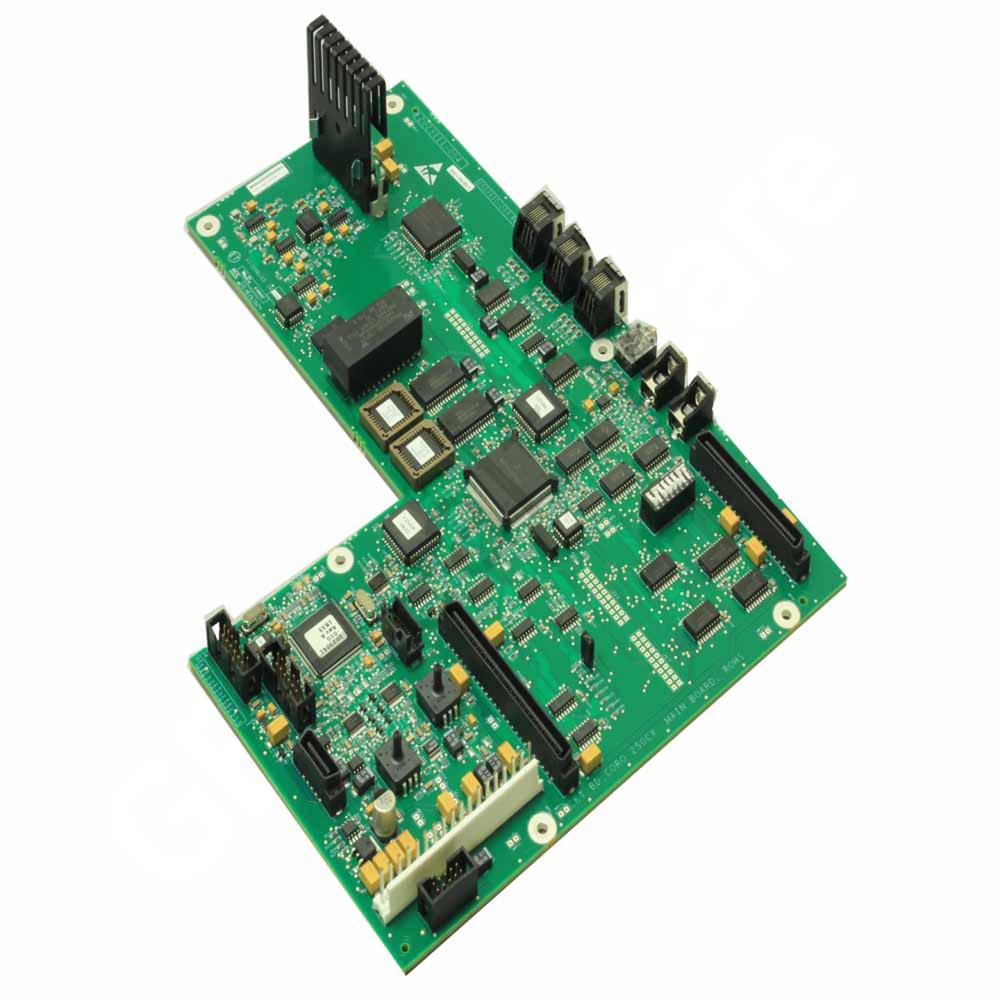
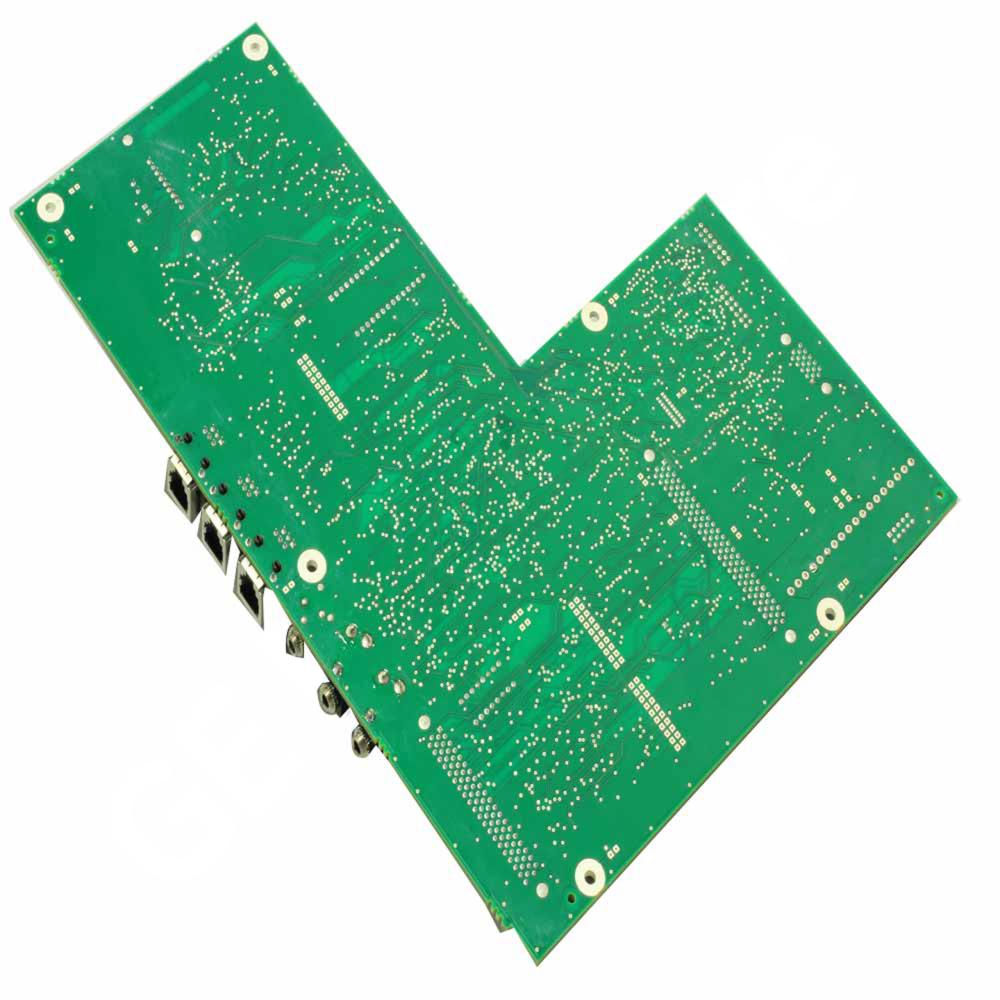
FRU, CORO250CX, MAIN BD,ROHS
| 2025177-078 | |
| 2025177-004 2025177-037 2025177-081 | |
| Maternal Infant Care | |
| GE Sistemas Médicos de México S.A. de C.V | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Optimized maintenance kit
- Packaging adheres to global specifications and is free of any physical damage
- Chemical resistant
- Environmental contamination free materials used
Product Overview
The FRU, CORO250CX, MAIN BD, ROHS are used in Patient Monitoring and other medical equipments as applicable. The part is a Field Replaceable Unit (FRU) during servicing and maintenance of display assembly units of Corometrics™ 250CX main board. It is a board which comprises of resistors, capacitors, IC’s, transistor, phone jack, connector jack, socket and diodes. The part is ROHS compliant and approved for today's safety standards. The product is a proven design which fits well into demanding applications. It is made from the high-quality material helps to offer a reliable operation, efficient functioning and increased lifespan. The GE product is an innovation and technology which fits well into versatile customer needs. The product is securely packaged inside a high quality packing box to avoid physical damage during transit and labeled with details about the product, Quality Assurance (QA) seal and shipment details.
Additional Features
- It is a ROHS compliant product
Equivalent Item(s):
We have the following equivalent items available for you.These are compatible replacements items.Those parts without a hyperlink are listed for reference only and are not available for purchase online.For additional information,please contact customer service
| Equivalent Item(s) | Item Details |
|---|---|
| 2025177-004 | FRU, CORO 250 , MAIN BOARD |
| 2025177-037 | FRU, CORO250CX, MAIN BD |
| 2025177-081 | • FRU, CORO 250 , MAIN BOARD,ROHS |